Vitasweet® APM
Production name:Vitasweet® APM
English name: Vitasweet, APM
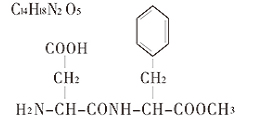
Chemical name: N-L-α-Aspartyl-L-Phenylalanine-1-Methyl Ester
Chemical structure:
Chemical formula:C14H18N2O5
Characteristics:
Intensive sweetness,200 times as sweet as sucrose in 3% solution
Clean sweetness without bitter, chemical ormetalic after-taste
Improving the taste profile
Effectively reduce the amount of calories in food
Used safely in any food and drink
Long-lasting perceivable sweet taste
Readily blending with most sweeteners to obtain
Ideal synergistic effect
Vitasweet® APM
In 1965,Aspartame was discovered
In 1981,FDA approved the use of Aspartame as tabletop sweeteners and flavor-improvers.
In 1981,Canada approved the use Aspartame in foods and beverage
In 1983, FDA approved the use of Aspartame in carbonated soft drinks.
In 1983, U.K. approved the use of Aspartame.
In 1986, China approved the use of Aspartame.
In 1988, Brazil approved the use of Aspartame.
In 1993, Aspartame was approved of use in confectioneries, baked foods, low-alcohol beer and all remaining nonalcoholic beverages.
In 1996, FDA approved Aspartame as a “general purpose” sweetener.
ADI of Aspartame set by FAO/WHO JECFA:40mg/kg body weight
ADI of Aspartame set by FDA: 50mg/kg body weight

Applications of APM in beverages:
Carbonated beverage | Drinks with coco flavor |
Citric acid | 6g | Whey powder | 53% |
Malic acid | 15g | Skim milk power | 25% |
Sodium fumarate | 3g | Coco | 18% |
Flavor | 0.03g | Sodium caseinate | 1% |
Vitasweet®APM | 18.87g | Salt | 0.5% |
Carbon dioxide water | To 30L | Carboxymethyl cellulose | 1% |
|
| Vitasweet®APM | 0.75% |
|
| Vanillin | Optimal dosage |
Applications of APM in food:
Gum | Chocolate |
Colloid makings | 26% | Chocolate liquor | 20% |
Sorbitol powder | 52% | Skim milk | 21% |
Sorbitol solution | 16% | Lactose | 37.7% |
Glycerin | 4% | Cocoa butter | 20% |
Flavor | 1.5% | Lecithin | 0.5% |
Vitasweet®APM | 0.5% | Vitasweet®APM | 0.2% |
|
| Flavor | 0.6% |
Application of Aspartame for the sweeteners in the table:
Recipe1 | Recipe2 |
Vitasweet®APM | 180g | Vitasweet®APM | 216g |
Filler | 588g | Sodium carbonate | 77g |
Carboxymethyl cellulose | 30g | Arabic gum | 15g |
Auxiliary tablet | 2g | Dehydarted enzyme sodium citrate | 70g |
Distilled water | 250ml | Tartaric acid | 316g |
|
| Calcium benzoate | 87g |
|
| Distilled water | Optimal dosage |
Aspartame is a dipeptide obtained by formal condensation of the alpha-carboxy group of L-aspartic acid with the amino group of methyl L-phenylalaninate. Commonly used as an artificial sweetener. It has a role as a sweetening agent, a nutraceutical, a micronutrient, a xenobiotic, an environmental contaminant, an apoptosis inhibitor and an EC 3.1.3.1 (alkaline phosphatase) inhibitor. It is a dipeptide, a carboxylic acid and a methyl ester. It derives from a L-aspartic acid and a methyl L-phenylalaninate.
Flavoring agent sweeter than sugar, metabolized as phenylalanine and aspartic acid.
IUPAC
(3S)-3-amino-4-[[(2S)-1-methoxy-1-oxo-3-phenylpropan-2-yl]amino]-4-oxobutanoic acid
SMILES
COC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](CC(=O)O)N