Ammonium sulphate
Ammonium sulphate is a kind of quick-acting fertilizer, which can be used for general crops, which can be used for recovery, fertilizer, base fertilizer and sulfur-lacking soil. However, the acid soil should be used in conjunction with lime (not mixed).In addition, it can also be used as a solder, fabric fireproofing agent, salt analysis process of pharmaceutical production, catalyst for food pickles, yeast culture, acid dyestuff staining, and leather dusting agent.It is easy to cause soil junction on neutral and rocky soil. The long-term use of ammonium sulfate will increase soil acidity.
Product Packaging, Handling and Storage
Packed in polypropylene woven bag lined with plastic film bag (or inside plastic film). Handle gently to prevent breakage.
In outdoor storage, it should pay attention to moisture, rain, avoid storage under high temperature, nor mixed with alkaline storage, water can be poured when the fire. Should pay attention to moistureproof during outdoor storage, prevent rain, avoid to store at high temperature, also do not mix with alkaline substance to store, if got fire could quench by water.
Product Characteristics
Ammonium sulphate’s abbreviation is SA, molecular formula: (NH4) HSO4, soluble in water, insoluble in ethanol. Aqueous solution with spicy salty taste, ammonium sulfate decomposition temperature is 280 ℃, release ammonia into NH4HSO4 ammonium sulfate acid type, the change of temperature of ammonium sulphate in water solubility, itself relative hygroscopicity is smaller.
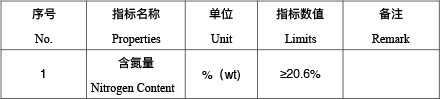
Ammonium Sulfate (SA) Standard GB/535-1995
Ammonium Sulphate Specification Table

Ammonium sulfate, also known as ammonium sulfate, is the earliest nitrogen fertilizer produced and used at home and abroad. It is usually regarded as standard nitrogen fertilizer, and the nitrogen content is between 20% ~ 30%. Ammonium sulfate is a strong acid weak alkali salt, and the aqueous solution is acidic. Ammonium sulfate is a kind of nitrogen fertilizer and acid fertilizer in inorganic fertilizer. It is used alone for a long time, which makes the soil acidified and hardened and needs to be improved. Ammonium sulfate cannot be used to produce organic fertilizer. Moreover, acid fertilizer can not be used with alkaline fertilizer, and double hydrolysis is easy to lose fertilizer efficiency. In the 1960s, ammonium sulfate was the main variety of nitrogen fertilizer and one of the main sources of elemental sulfur providing crop nutrition . It was first made by neutralization with ammonia and sulfuric acid, but later the proportion of by-product ammonium sulfate became larger and larger. Now, all domestic by-products basically come from other industries, such as coking industry, caprolactam, sulfuric acid tail gas desulfurization, power plant desulfurization, acrylonitrile, methyl methacrylate, zinc oxide and other by-products. The by-product ammonium sulfate follows the principle of "treating waste with waste", realizes the comprehensive utilization of waste and achieves the purpose of energy conservation and emission reduction. Especially with the breakthrough of ammonia desulfurization project technology, the output of ammonium sulfate, the by-product of desulfurization in power plant, will increase significantly in the future.
SMILES
[NH4+].[NH4+].[O-]S(=O)(=O)[O-]