Thiamethoxam Tech
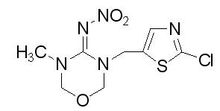
Structure:

Synonymous:Aktai; 3 - (2-chloro-1,3-thiazol-5-ylmethyl) - 5-methyl-1,3,5-oxadiazine-4-ylidene (nitro) amine
Molecular Formula:C8H10ClN5O3S
Molecular weight:291.71
Toxicity: low toxicity
Melting point:139.1℃
Water solubility:4.1g
Steam pressure: 6.6×10-9Pa(25℃)
Appearance: white crystalline powder
Product specification: 98%
Content and dosage form: 25% water dispersible granules, 50% water dispersible granules and 70% seed treated dispersible granules
Application characteristics: thiazoxam is a new generation of second generation nicotine type high efficiency and low toxicity insecticide. It has gastric toxicity, contact toxicity and internal sucking activity. It is used for foliar spray and soil irrigation. After application, it is rapidly absorbed and transmitted to all parts of the plant. It has a good control effect on thorn sucking pests such as aphids, planthoppers, leaf cicadas and whiteflies.
There was no cross resistance with imidacloprid, acetamiprid and acetamiprid. It can be used for stem and leaf treatment, seed treatment and soil treatment. Suitable crops are rice crops, sugar beet, rape, potato, cotton, kidney beans, fruit trees, peanuts, sunflowers, soybeans, tobacco and citrus. Use at the recommended dose is safe and harmless to crops.
Packing specification: 25kg / barrel, 50kg / barrel (can also be customized according to customer requirements)
Actara is a new broad-spectrum insecticide with high efficiency and low toxicity. It is the second generation of new nicotine insecticides. Its action mechanism is similar to that of the first generation of new nicotine insecticides such as imidacloprid, but it has higher activity. Actara has the characteristics of gastric toxicity, contact killing, internal absorption, fast action speed and long duration. It has a good control effect on thorn sucking pests such as aphids, planthoppers and whiteflies.
IUPAC
(NE)-N-[3-[(2-chloro-1,3-thiazol-5-yl)methyl]-5-methyl-1,3,5-oxadiazinan-4-ylidene]nitramide
SMILES
CN1COCN(C1=N[N+](=O)[O-])CC2=CN=C(S2)Cl