
 Hubei, China
Hubei, China
 Hubei, China
Hubei, China
Send Email






PQQ Disodium Salt / Methoxatin Disodium Salt / PQQ-2Na / CAS 122628-50-6
OVERVIEW
Benefits
The company has strong R&D strength (the research background of Angel Yeast and East China Medicine), and the quality standard conforms to USP.
The production capacity is stable, with an annual capacity of 20T.
Flexible production, providing OEM and non-standard customized services.
Operating of ISO9001, ISO22000, ISO14001, OHSAS18001 , HACCP, and other management systems.
BRC Global Food Safety, Ethical Audit, Kosher, Halal Certification, etc.
No syntheic process; No additives and preservatives; NON-GMO; Fermented from patented strains; No bacterial and solvent residues; Unique crystal form; Unique patented sustained release technology.
Specification
Item | Specification | Result |
Appearance | Henna powder | Conforms |
Identification | Positive | Conforms |
Purity(on dry basis) | ≥98% | 99.40% |
Loss on Drying | ≤12% | 8.90% |
Arsenic | ≤10 ppm | Conforms |
Lead | ≤1.5 ppm | <0.1 ppm |
Mercury | ≤1ppm | 0.3 ppm |
Cadmium | ≤0.2 ppm | <0.1 ppm |
Chromium | ≤lppm | <0.1 ppm |
Nickel | ≤1ppm | <0.1 ppm |
Total bacterial count | ≤ 10,000 CFU/g | 580 CFU/g |
Mold & Yeast | ≤300 CFU/g | 45 CFU/g |
Escherichia coli | Absent | ND |
Staphylococcus aureus | Absent | ND |
Salmonella | Absent | ND |
Uses
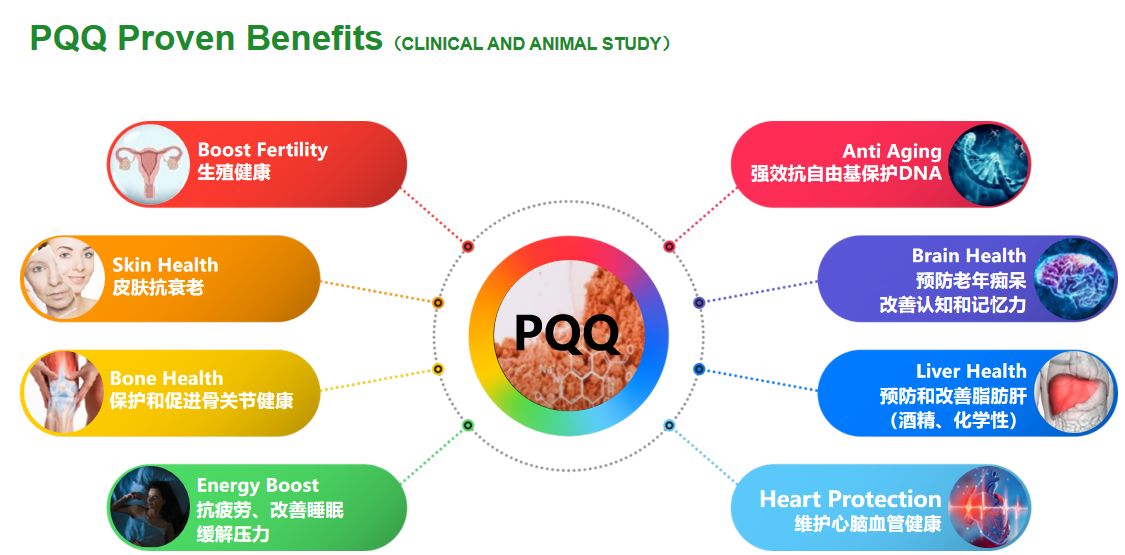
Boost Fertility
Reproductive health
Skin anti-aging
Protecting and promoting bone and joint health
Anti fatigue, improves sleep
relieve stress
Strong anti free radical protection of DNA
Preventing Alzheimer's disease
Improving cognition and memory
Prevention and improvement of fatty liver disease (alcohol, chemical)
Maintain cardiovascular and cerebrovascular health
Packaging
| Pallets Packing | 20'FCL | 40'FCL | ||
| With | Without | With | Without | |
|
10 KG/Carton
Purity(on dry basis)≥98%Pharm Grade USP
|
--
|
--
|
--
|
--
|
|
10 KG/Carton
Purity(on dry basis)≥98%Food Grade
|
--
|
--
|
--
|
--
|
Lead Time
In Stock
Max Capacity
20 MT/Year
REQUEST A QUOTATION
SubmitDESCRIPTION
Highlights of Magic Health Technology PQQ Disodium Salt:
1. The company has strong R&D strength (the research background of Angel Yeast and East China Medicine), and the quality standard conforms to USP.
2. The production capacity is stable, with an annual capacity of 20T.
3. Flexible production, providing OEM and non-standard customized services.
4. Operating of ISO9001, ISO22000, ISO14001, OHSAS18001 , HACCP, and other management systems.
BRC Global Food Safety, Ethical Audit, Kosher, Halal Certification, etc.
Quick Details
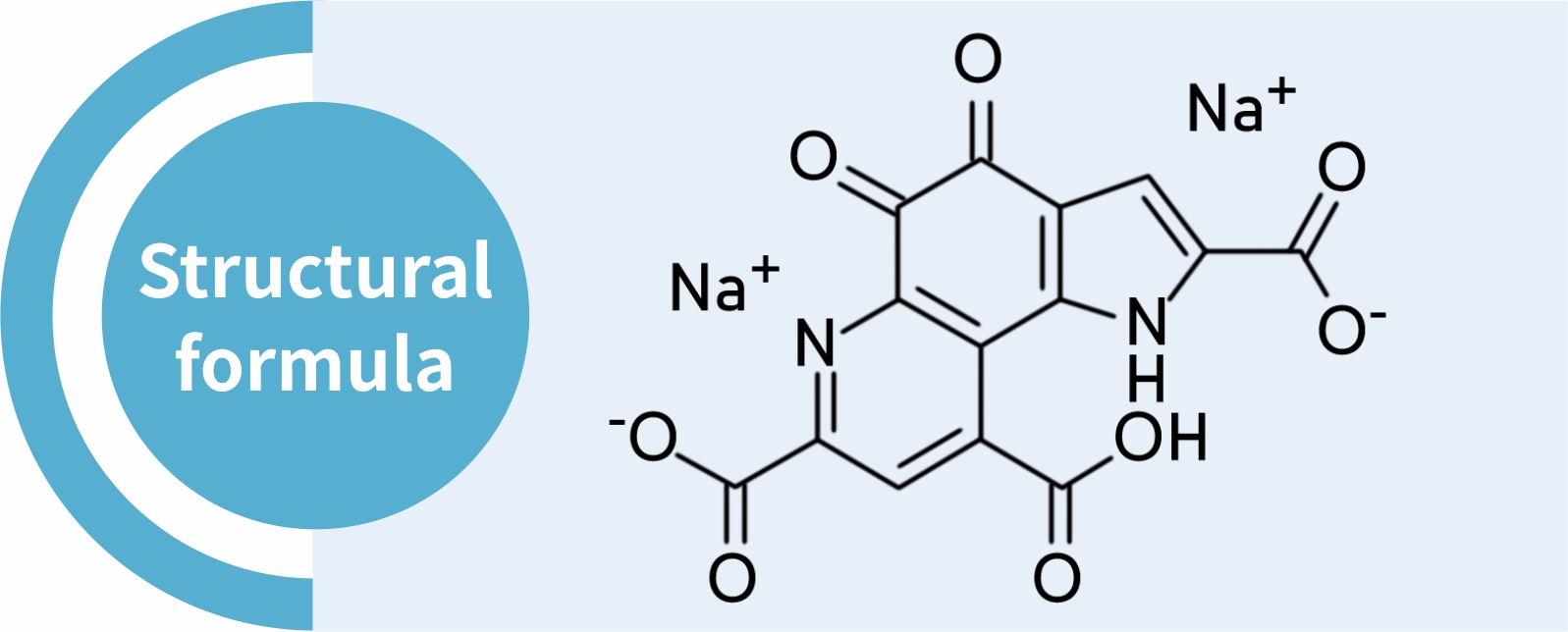
Product Name: | Pyrroloquinoline Quinone Disodium Salt | CAS No.: | 122628-50-6 |
MF: | C14H4N2Na2O8 | Abbr: | PQQ.2Na; PQQ-2Na |
Other Names: | Methoxatin Disodium Salt; PQQ Disodium Salt; Pyrroloquinoline Quinone.2Na; Pyrroloquinoline Quinone Disodium Salt Powder; | Type: | Food; Health Care Products; Medicine; Cosmetics; |
Grade Standard: | Food Grade; Pharm Grade | Purity: | ≥98%/USP/CP ;≥99% |
Transport Package: | Al foil bag/Box | Shelf Life: | 24 Months |
Appearance: | The reddish-brown crystalline powder | Origin: | China |
Application: | Pyrroloquinoline Quinone Disodium Salt is an effective component of nutritional ingredients currently sold in the market. It has nerve growth factor-inducing activity, has certain effects on Parkinson's disease, and can prevent immune liver injury | ||
Supply Ability
20 Metric Tons Per Year
Packaging & Delivery
Packaging Details: | 10 KG/Box, with the inner aluminum foil bag or according to customer requirements |
Port: | Shanghai Port, or other main ports in China |
Specifications of Pyrroloquinoline Quinone Disodium Salt CAS 122628-50-6
Item | Specification | Result |
Appearance | Red or reddish-brown powder | Reddish-brown powder |
Identification | HPLC: Conforms to the reference solution | Conforms |
IR: Conforms to RS | Conforms | |
Water | ≤12.0% | 9.00% |
Related substances | Any individual impurity≤0.1% | <0.05% |
Total impurities≤1.0% | <0.05% | |
Assay(PQQ disodium salt calculated on dry basis) | 98.0%~102.0% | 99.90% |
Sodium content (on dry basis) | 10.5%~12.9% | 12.20% |
Arsenic | ≤ 0.2 mg/kg | 0.2 mg/kg |
Lead | ≤ 0.2 mg/kg | 0.2 mg/kg |
Mercury | ≤ 0.2 mg/kg | 0.2 mg/kg |

Hubei Magic Health Technology Co., Ltd is established in 2021, jointly funded by Hangzhou Zhongmei Huadong Medicine Co., Ltd, Zhejiang Huida Biotech Co., Ltd, and Angel Yeast Co., Ltd.
● Specializing in the R&D and manufacturing of nutraceutical food raw materials and personal care functional raw materials based on microbial technology.
● Located in Biological Industrial Park, Xiaoting District, Yichang City, Hubei, China. Total covered area: 148,000㎡, total construction area: 164,000㎡ , total planned investment: 1.16 billion RMB(for three phases.
● It has given full play to the sources and advantages of the three shareholders in product R&D, technological innovation, manufacturing, quality certification and other aspects, and is committed to being an "industrialized, large-scale and international" technical innovation company with the comprehensive health industry being the backbone force.

R & D
- Development and Research on Microbial Sources of International Frontier Health Products
- Focuses on the development, research and technological upgrading of major health-related products, with nearly 40 reserve projects.
- Obtained and applied for a number of international and domestic patent research and development achievements.
- Covering large health products strain selection, fermentation engineering, purification and separation, instrument analysis, quality research, terminal application research
Research direction:
1. Major health areas: vitamin K2, PQQ, hyaluronic acid, etc;
2. Protection areas: Icodoin, hyaluronic acid, ergothioneine, etc;
3. Technical cooperation.
PQQ is the strongest antioxidant in nature, the oxidation resistance is 180 times higher than vitamin C.
Magic Health was jointly founded by Angel Yeast and Huadong Medicine, which specialized in manufacturing naturally fermented functional food raw materials like Vitamin K2, PQQ, etc., and functional personal care raw materials like Ectoin, and ergothioneine(EGT).
Description of Pyrroloquinoline Quinone Disodium Salt CAS 122628-50-6
Pyrroloquinoline quinone, PQQ for short, is a new coenzyme different from nicotinamide nucleotides (NAD + and NADP +) and flavin nucleotides (FAD and FMN). PQQ is a cofactor of dehydrogenase redox reaction in bacteria. In 1979, Durine and other researchers separated the coenzyme from bacteria, and then its structure was confirmed as 4,5-dihydro-4,5-dioxo-1-hydropyrrolo (2,3-f) quinoline-2,7,9-tricarboxylic acid.
PQQ is widely distributed in nature from microorganisms to animal tissues, but it is considered that only Gram-negative bacteria can synthesize PQQ, Human beings can only obtain PQQ through diet to meet the needs of the body, and the intestinal flora cannot synthesize PQQ. Therefore, exogenous PQQ intake is very important to maintain the needs of human and animal tissues and the stability of intestinal flora.
PQQ has high water solubility and thermal stability. Under neutral or weak alkaline conditions, its carboxyl group can be dehydrogenated into an anionic state. Current research shows that the form of PQQ disodium salt is most easily absorbed by the human intestine. PQQ has three forms: quinone type (oxidation type), semiquinone type, and hydroquinone type (PQQH2), which are transformed into each other through electron and proton transfer. The stable chemical structure and high redox potential make PQQ the bioactive molecule with the strongest ability to catalyze redox reactions so far.
Efficacy and Mechanism of Action of Pyrroloquinoline Quinone Disodium Salt CAS 122628-50-6
●Antioxidant Mechanism
The production and elimination of various free radicals in organisms should be in dynamic balance. Too many or too few free radicals are unfavorable to the body. Too much will promote the aging of the body and lead to various diseases, including cancer, heart disease and so on.
When too little, it will also affect health, even hinder normal metabolism, or induce another kind of disease. In modern society, due to environmental deterioration and irregular diet, the accumulation of free radicals and harmful substances in the human body is becoming more and more serious. PQQ, as a redox coenzyme, its special quinone structure can prevent oxidative damage through the following mechanisms.
PQQ can catalyze the mutual conversion of oxygen and O2, so as to help the body maintain free radical balance.
O2+e-+H+⇋[PQQ↔PQQH2]⇋O2-+H+
PQQ combines with superoxide dismutase (SOD) to form a broad-spectrum oxidase system that produces hydrogen peroxide, so as to inhibit the damage of superoxide anion free radicals to cells. Sod is used as an apoenzyme protein and PQQ is used as non covalently bound redox accessory.
2O2+2e-→2O2-
2O++NAD++H+(PQQ coenzyme)→NADH+2O2
By accelerating the reaction of "NAD + → NADH", PQQ can convert the utilized oxidized glutathione (GSSG) to reduced glutathione (GSH) faster.
NAD++H+GSSG(GSSG reductase)→2GSH+NAD+

●Protect the Heart from Oxidative Impairment
PQQ can eliminate reactive oxygen species (ROS) produced by hypoxia reperfusion and significantly reduce the release of lactate dehydrogenase in the heart. Under the catalysis of flavin mononucleotide reductase, its catalytic product can also reduce the peroxidation state of hemoglobin and eliminate the damage of myocardial ischemia reperfusion.
Studies have shown that PQQ can protect the heart of ischemia-reperfusion mice, significantly reduce the scope of myocardial infarction, enhance the rise and fall rate of left ventricular pressure and left ventricular diastolic pressure, reduce ventricular fibrillation and reduce the level of malondialdehyde in myocardial tissue.
PQQ can also inhibit the production of ROS in rat cardiomyocytes induced by hydrogen peroxide and the decrease of mitochondrial membrane potential, so as to reduce oxidative stress, inhibit the inactivation of mitochondrial function and protect rat cardiomyocytes.
●Prevention of Hepatic Impairment
The liver is the main metabolic and detoxifying organ of animals and the human body. In the research, researchers found that the experimental liver injury of rats caused by carbon tetrachloride (Ccl4), galactosamine
thioacetamide and other toxins can be prevented by injecting a certain dose of PQQ and its derivatives into the abdominal cavity in advance.

PQQ can reduce the productionofROScaused by liver toxic substances, significantly reduce the levels of serum bilirubin glutamic pyruvic transaminase(GPT)and lactate dehydrogenase block liver cell necrosis and do not affect the routine biochemical indexes of rats(such as blood glucose, hematuria nitrogen, etc.).
●Promote Nerve Growth and Protect the Nervous System
Nerve growth factor (NGF) is the earliest and most thoroughly studied neurotrophic factor. It has the dual biological functions of neuronal nutrition and neuroprotection. It plays an important regulatory role in the growth, development, differentiation, regeneration, and biological function-specific expression of central and peripheral neurons. The results showed that PQQ could stimulate L-M cells and Schwann cells to produce NGF in vitro.
●Prevent Acetaldehyde Poisoning
Acetaldehyde is the intermediate metabolite of alcohol in animals, which is toxic. Many people have acetaldehyde dehydrogenase gene mutation and incomplete function, resulting in acetaldehyde accumulation after drinking, resulting in slight acetaldehyde poisoning reactions such as blushing and dizziness.
Studies using rodents have found that PQQ contributes to the metabolism of acetaldehyde. PQQ (11.5mg/kg body weight) was injected intraperitoneally before ethanol was injected into the stomach. It was found that there was no significant difference in ethanol concentration in blood and liver between the treatment group and the control group, but the acetaldehyde concentration of the former was much lower than that of the latter. Replacing PQQ with other quinone derivatives such as coenzyme Q10 has a similar effect.

Regulatory Dosage and Usage of Pyrroloquinoline Quinone Disodium Salt CAS 122628-50-6
The safety study in animal
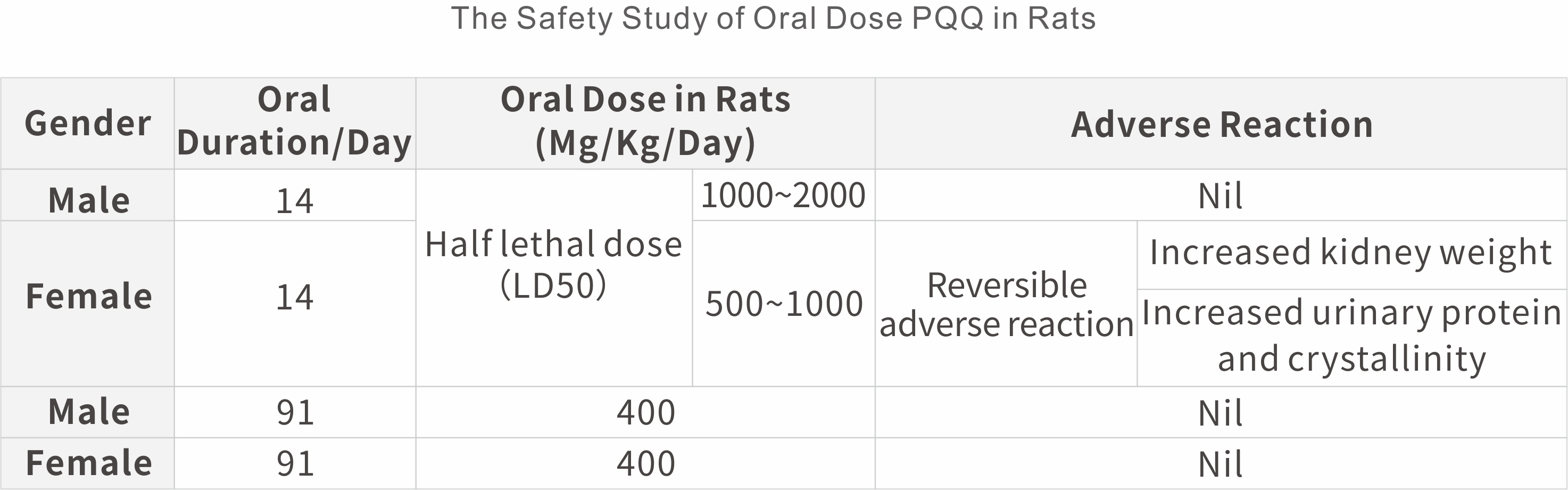
In the human-controlled double-blind clinical trial, volunteers took PQQ at 60 mg/day for one month, and there were no adverse reactions and no changes in renal injury markers. The safe dose of FDA is: healthy about 60kg treated with a dose of 240mg PQQ every day, and there are few adverse events.
USFDA recommended the use scope and maximum use level of PQQ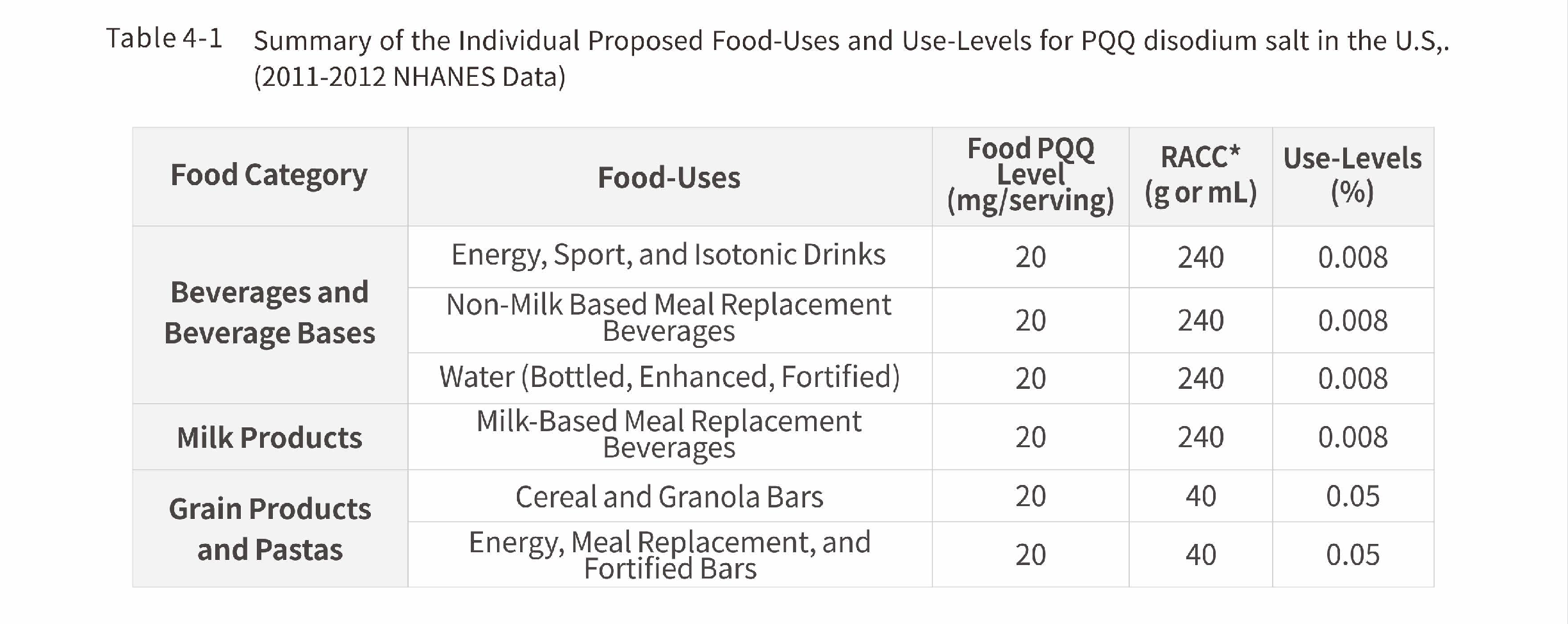
*RACC = Reference Amounts Customarily Consumed per Eating Occasion (21 CFR $101.12 - CFR, 2014b). When ar ange of values is reported for a proposed food-use, particular foods within that food-use may differ with respect to their RACC.
USFDA proposed daily intake of PQQ by population group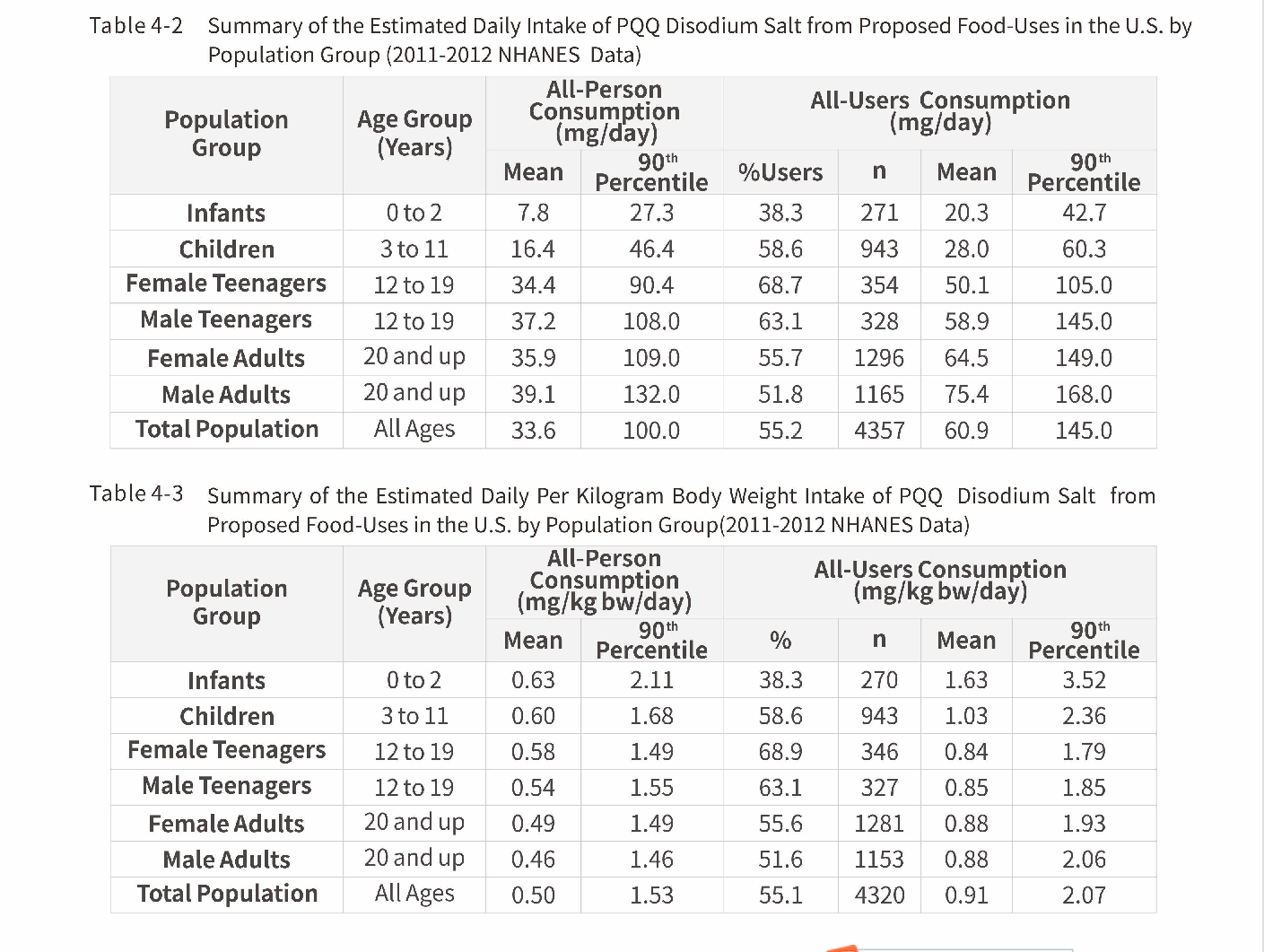
Shelf life
Store in a cool, dry and airy place, protect from light, with a shelf life of 24 months.
Production and quality control
Angel yeast Co., Ltd. was founded in 1986, specializing in the processing and manufacturing of yeast and yeast derivatives. Its production and marketing scale ranks first in Asia and third in the world. It has established a global marketing network, regional headquarters and application technology service centers in Beijing, Shanghai, Chengdu, Shenyang, Wuhan, Guangzhou, Cairo and other cities, and products are exported to more than 150 countries and regions.
Angel yeast Co., Ltd. has passed ISO9001 quality management system certification, HACCP certification, GMP certification and kosher certification, and the testing center has been recognized by CNAs, providing a reliable guarantee for the production of products.

Keywords Related to Pyrroloquinoline Quinone Disodium Salt CAS 122628-50-6
Pyrroloquinoline Quinone Disodium Salt; CAS122628-50-6; Pyrroloquinoline Quinone; PQQ.2Na; PQQ-2Na; PQQ Disodium Salt; PQQ Powder; Pyrroloquinoline Quinone Powder; China Pyrroloquinoline Quinone Powder; Pyrroloquinoline Quinone Disodium Salt Manufacturers; China Pyrroloquinoline Quinone Disodium Salt; Pyrroloquinoline Quinone Manufacturers; Pyrroloquinoline Quinone 99%; Pyrroloquinoline Quinone Suppliers; PQQ.2Na Powder; Pyrroloquinoline Quinone PQQ; Pyrroloquinoline Quinone Disodium Salt 99%; Pyrroloquinoline Quinone Disodium Salt Powder; Pyrroloquinoline Quinone Disodium Salt Suppliers; PQQ-2Na Powder.
FAQ of Magic Health Technology
Q1. Are you a manufacturer or trading company?
We are a manufacturer that owns factories both in Hubei Province. They cover an area of about 323 mu. You are warmly welcome to visit us!
Q2. What are your payment terms and types?
Payment Type: L/C, T/T, D/P, OA, Paypal, Western Union.
Q3. We don’t know you at all, how can we trust you?
You are always warmly welcome to visit us at any time. Before we start B2B, Chemball has audited our company and approved our credit. Commitment is the No.1 Point of our Enterprise’s Value.
Q4. How to contact us?
Contact us by Chemball page, email, mobile phone, or any way you like! We will try our best to respond ASAP!
Typical Properties
API & Intermediate > Vitamins And Minerals Medicines
GET SAMPLE
Submit- Overview
- Documents
- Descriptions
- Sample












