Sodium Carboxyl Methylstarch
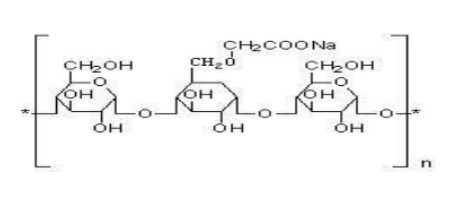
Structure:
Molecular formula:(C3H5ClO⋅C2H7N⋅H3N)×
Approval No.: LYZZ f2014028
CDE Registration No.: 220209990419
Quality standard: CP
Project 2020 Edition Indicators
Identification ; Meets the requirements
PH: 5.5 ~ 7.5
Sodium chloride% < 6.0
Sodium glycolate% ≤ 2.0
Loss on drying% ≤ 10.0
Iron salt% ≤ 0.002
Heavy metal% ≤ 0 002
Chloroacetic acid < 0.2
Content (sodium)% 2.0 ~ 4.0
Microbial limit ;Meets the requirements
Character:
This product is white or off white powder.
This product is insoluble in ethanol.
Category:
Pharmaceutical excipients, disintegrating agents and fillers, etc.
Purpose:
This product is a derivative of starch and is mainly used as a pre hydrolysate of solid preparations. It has good water absorption and expansion. It has compressibility while accelerating the tablet and spray dissolution. It can improve the or pinch of the tablet and increase the tablet properties. The dosage is 0.5 ~ 8% and the common dosage is 3%. The application method is generally external or internal and external addition.
Packing:
The pearlescent film outer bag is lined with polyethylene film bag, and the net weight is 25kg / bag.
Storage:
Seal and store in a dry place.
Indication: ① the source and model of starch raw material of this product shall be indicated. ② The particle size and particle size distribution of the product shall be indicated. ③ Type A, B and D shall indicate the marked value or range of expansion volume. Note: This product has hygroscopicity.
The utility model relates to a water-soluble anionic polymer compound, which is used as an additive in suspension, stability, warp sizing agent, dyeing assistant, etc. in water-based coatings
Used as pharmaceutical excipients and excipients