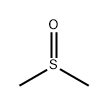
Dimethyl Sulfoxide
Name: Dimethyl Sulfoxide
Structural type: 
Molecular formula: C2H6OS
Molecular weight: 78.1334
CAS:67-68-5
Chemical properties: colorless liquid with hygroscopicity. Almost odorless, with a bitter taste. Soluble in water, ethanol, acetone, ether, benzene and chloroform.
Uses: as organic solvent, reaction medium and intermediate of organic synthesis. It has a wide range of uses. With high selective extraction ability, it can be used as polymerization and condensation solvent of acrylic resin and polysulfone resin, polymerization and spinning solvent of Polyacrylonitrile and acetic acid fiber, extraction solvent for the separation of alkanes and aromatics, reaction medium for aromatics and butadiene extraction, acrylic fiber spinning, plastic solvent, organic synthetic dyes, pharmaceutical and other industries. In medicine, it has anti-inflammatory and analgesic effects and strong penetration into the skin.
Packing: plastic barrel, net weight 200 kg per barrel.
Dimethyl Sulfoxide is a highly polar organic liquid that is used widely as a chemical solvent and a free radical scavenger. It shows a range of pharmacological activity including analgesia and anti-inflammation. Because of its ability to penetrate biological membranes, it is used as a vehicle for topical application of pharmaceuticals. It is also used to protect cells and tissue during cryopreservation and has been used to treat extravasation damage caused by anthracycline-based chemotherapy.
Dimethyl sulfoxide appears as a clear liquid, essentially odorless. Closed cup flash point 192°F. Vapors are heavier than air. Contact with the skin may cause stinging and burning and lead to an odor of garlic on the breath. An excellent solvent that can transport toxic solutes through the skin. High vapor concentrations may cause headache, dizziness, and sedation.
IUPAC
methylsulfinylmethane