Copper(II) Sulfate
CAS RN:7758-98-7
EINECS NO.:231-847-6
Molecular Formula:CuO4S
Molecular Weight:159.60
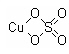
Structural Formula:

Properties:
Density:1.04 g/cm3
M.P.:560°C (dec.)
Sol. in Water:203 g/L (20°C)
Range of Application:It is used in the manufacture of other salts such ascuprous chloride,copper chloride,copper pyrophosphate,cuprous oxide,copper acetate,copper carbonateetc.; or in the production of monoazo dyes like ac reactive brilliant blue, reactive violet etc.; in the synthesis of perfumes and dyes intermediate; as mordant and pro-oxygenic agent; as bactericide.
Health Hazard:It is highly irritating to gastrointestinal tract, and can cause nausea, vomit and stomach burning or even angina abdominis, hematemesis, hemolysis etc. By long-time contact, it can also lead to contact dermatitis.
Skin Contact:Take of clothes, and wash with plenty of flowing water.
Eye Contact:Wash eyes with flowing water or physiological salinephysiological saline; see the doctor.
Inhalation:Evacuated from the site to places with fresh air. If decompensation appears, take oxygen therapy and see the doctor.
Ingestion:Take gastric lavage with 0.1% yellow prussiate of potash or sodium thiosulfate; drink milk or egg white; see the doctor.
Operation:Air-tight environment, and provide sufficient local air discharge. Operators must be strictly trained and obey related operating instruction. Self-inhalation filtertype dust respirators, rubber gloves, protective goggles should be worn. Avoid dust, acids or alkalis. Handle with care; prevent?damage to packing and containers.
Storage:Stored in cool, dry and draughty warehouse; kept away from fire or heat source. Keep containers air-tight. No mixing with acids, alkalis and food chemicals.
Copper sulfate, also known as blue alum, with chemical formula CuSO4, is white powder without water, blue powder with water, or light grayish green due to impure. It is a soluble copper salt. The common form of copper sulfate is its crystal. Copper sulfate tetrahydrate ([Cu (H2O) 4] SO4 · H2O, copper sulfate pentahydrate) is a blue solid. Its aqueous solution is blue due to hydrated copper ions, so anhydrous copper sulfate is often used to test the existence of water in the laboratory. In real production and life, copper sulfate is often used to refine refined copper, which can be mixed with hydrated lime to produce pesticide Bordeaux liquid. Copper sulfate is a heavy metal salt, toxic, and the lethal dose for adults is 0.9g/kg. If you eat by mistake, you should immediately eat or drink protein rich foods such as milk and egg white, or use EDTA calcium sodium salt to detoxify. White anhydrous copper sulfate powder is blue after dropping into water
SMILES
[O-]S(=O)(=O)[O-].[Cu+2]