b

Thank You!
Your requirement has been sent, we will contact you quickly!
Sent Failed!
Try again!
Ammonium sulphate is a kind of quick-acting fertilizer, which can be used for general crops, which can be used for recovery, fertilizer, base fertilizer and sulfur-lacking soil. However, the acid soil should be used in conjunction with lime (not mixed).In addition, it can also be used as a solder, fabric fireproofing agent, salt analysis process of pharmaceutical production, catalyst for food pickles, yeast culture, acid dyestuff staining, and leather dusting agent.It is easy to cause soil junction on neutral and rocky soil. The long-term use of ammonium sulfate will increase soil acidity.
Product Packaging, Handling and Storage
Packed in polypropylene woven bag lined with plastic film bag (or inside plastic film). Handle gently to prevent breakage.
In outdoor storage, it should pay attention to moisture, rain, avoid storage under high temperature, nor mixed with alkaline storage, water can be poured when the fire. Should pay attention to moistureproof during outdoor storage, prevent rain, avoid to store at high temperature, also do not mix with alkaline substance to store, if got fire could quench by water.
Product Characteristics
Ammonium sulphate’s abbreviation is SA, molecular formula: (NH4) HSO4, soluble in water, insoluble in ethanol. Aqueous solution with spicy salty taste, ammonium sulfate decomposition temperature is 280 ℃, release ammonia into NH4HSO4 ammonium sulfate acid type, the change of temperature of ammonium sulphate in water solubility, itself relative hygroscopicity is smaller.
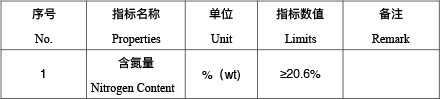
Ammonium Sulfate (SA) Standard GB/535-1995
Ammonium Sulphate Specification Table