b

Thank You!
Your requirement has been sent, we will contact you quickly!
Sent Failed!
Try again!
Product introduction:
Zinc hydroxide colorless orthorhombic crystal. Relative density 3.05. After drying at 40 ~ 50 ℃, it conforms to the molecular formula composition, and decomposes into zinc oxide and water at 125 ℃. Insoluble in water, soluble in strong acid to form zinc salt, soluble in strong alkali to form zinc salt, soluble in excess ammonia to form zinc ammonia coordination ion. Add an appropriate amount of sodium hydroxide solution to zinc nitrate solution and precipitate.
CAS:20427-58-1
Molecular formula: H2O2Zn
Molecular weight: 99.39
Structural type: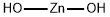
Physicochemical properties: Amorphous white powder or light yellow powder, relative density 3.053. The melting point is 125 ℃ (decomposition). It decomposes into zinc oxide at 120 ℃. It is insoluble in water, soluble in acid, alkali solution and ammonia. It is an amphoteric hydroxide.
Product purpose:
It is used to produce zinc compounds, such as zinc oxide, zinc sulfate, zinc nitrate, etc.
Used as analytical reagent, also used in rubber manufacturing and pharmaceutical industry.

