b

Thank You!
Your requirement has been sent, we will contact you quickly!
Sent Failed!
Try again!
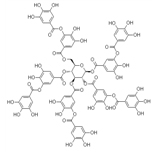
Structural formula:
Specification | Drug grade |
executive standard | USP34/CP2010/BP2012 |
content | ≥92% |
loss on drying | ≤12% |
water insoluble | ≤0.1% |
ignition residuce | ≤0.1% |
heavy metal | 40ppm |
production scale | 300 T/Y |
packing | Cardboard drum, 25kg/ drum |
Character: This product is light yellow to light brown powder, with special smell and astringent taste. Soluble in 1 part water or ethanol, soluble in acetone, insoluble in chloroform or ether.
Quality indicators: in line with the 2012 edition of the British Pharmacopoeia and the 2013 edition of the United States Pharmacopoeia.
To use: Medical tannic acid has antibacterial, deenzymatic, astringent effects, used in antibiotics, ringworm treatment, detoxification, diarrhea and other pharmaceutical preparations, but also used in the synthesis of sulfanilamide synergistic agent
Storage: keep away from moisture and light and store sealed
Packing: plastic inner and paper drums of 25 kg net per barrel

