b

Thank You!
Your requirement has been sent, we will contact you quickly!
Sent Failed!
Try again!
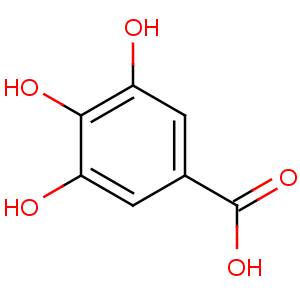
Molecular formula:
Production scale: 1500 tons/year
Synonyms: gallic acid
Chemical name: 3,4,5-trihydroxybenzoic acid
Molecular formula and molecular weight: C7H8O6; 188.13
Properties: white or grayish white crystalline powder, and could be dissolved in 85 shares of water, 6 shares of ethyl alcohol, 2 shares of boiling water, and could be slightly dissolved in the diethyl ether.
Quality index: Conforming to European Standard-93 (First Grade), Japanese National Standard (JIS) Standard (First Grade), 21st edition of USA Pharmacopoeia (Top-Class Product).
Uses: Widely used in such industries as pharmaceuticals, dyes, chemicals and organic synthesis, as well as in the analysis of rare metals.
Storage: Store in an airtight container.
Package: Inner plastic and outer woven bags, 25 kg net weight per bag.
Quality standard:
Content% | ≥99.0 |
Sulfate ppm | ≤10 |
Tannic acid test ppm | ≤1.0 |
Dry weightlessness% | ≤10.0 |
Burning residue% | ≤0.1 |
Turbidity ppm | ≤10 |
Chromatic number | ≤200 |

