Mycophenolate Mofetil (MMF)
1、Nomenclature
English name:Mycophenolate Mofetil (MMF)
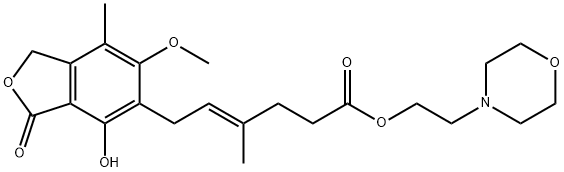
2、Structural formula
Molecular Structure |  |
Molecular formula:C23H31NO7
Molecular weight:433.50
CAS No.: 128794-94-5
3、Mechanism and clinical use:
Mycophenolate Mofetil is synthesized by Mycophenolic Acid and 4-(2-Hydroxyethyl) morpholine. It is immunosuppressive agent; inosine monophosphate dehydrogenase (IMPDH) inhibitor. It is used for the prophylaxis of organ rejection in patients receiving allogeneic renal, cardiac or hepatic transplants.
Mycophenolate Mofetil has more strong inhibition effect for EB virus induced B lymphoblast transformation. 1, When it is used in combination with other immunosuppressive drugs, it will increase the risk of lymphoma and other malignant tumor (especially skin cancer), and overinhibition to immune system may increase infection opportunity. 2, When it is used in combination with acyclovir or ganciclovir, there is no obvious change of blood concentration of MPA; when the combination used for patients with renal function impairment, blood concentration of acyclovir or ganciclovir gets to rise. 3, Sulfinpyrazone may interfere this drug’s secretion from kidney tubules, if MMF is used in combination with sulfinpyrazone, this drug’s toxicity is enhanced. 4, When MMF is used in combination with antacid agents which contains magnesium or aluminum (such as magnesium hydroxide and aluminium hydroxide), the absorption of this drug is to be reduced. 5, Echinacea purpurea may excite immune system to reduce this drug’s efficacy. 6, Ferrous drugs may reduce the absorption and efficacy of this drug. 7, If the drug is used in combination with drugs that may disturb enterohepatic cycling (such as colestyramine), may reduce efficacy. 8, It can not be excluded the possibility of changing pharmacokinetics parameters of oral contraceptives after long time administration. This may reduce efficacy of oral contraceptives. 9, During taking of this drug, vaccination of live attenuated vaccines should be avoided, and other vaccines may have no efficacy during the administration period of this drug.
On Dec. 2019, Mycophenolate Mofetil passed GMP re certification .
On Dec.2020, API Mycophenolate Mofetil was granted “written confirmation for active substance exported to EU”.
4、Packaging size specification
Inner package: double-layers polyethylene bag and aluminum foil bag.
Outer package: fiber drum.
Packaging size: 20 Kg/drum