b

Thank You!
Your requirement has been sent, we will contact you quickly!
Sent Failed!
Try again!
JK Sucralose Inc. is the only producer of pharmaceutical-grade sucralose in China and is the only manufacturer to obtain a license to produce sucralose for use in pharmaceutical products.
JK's products are in full compliance with FCCVII, USP34-NF29, EC231/2012(2008/60/EC), EP2368, and GB25531 quality standards, as well as Japanese Standards. JK is an approved qualified supplier of various multinational food and beverage giants.
Product Name: Sucralose
Chemical Name: 1',6'-Dichloro-1', 6'-dideoxy-β-D-fructofuranosyl-4-chloro-4-deoxy-α-D-galactopyranoside
Synonyms: TGS; 4, 1', 6'-Trichlorogalactosucrose; Trichlorogalactosucrose
CAS No.: 56038-13-2
Molecular Formula: C12H19O8Cl3
JK's products are in full compliance with FCCVII, USP34-NF29, EC231/2012(2008/60/EC), EP2368, and GB25531 quality standards, as well as Japanese Standards. JK is an approved qualified supplier of various multinational food and beverage giants.
Item | FCCVII | USP34-NF29 | JP | GB25531 | JK Standard |
Appearance | White crystalline powder | —— | White crystalline powder | White crystalline powder | White crystalline powder |
Identification | The retention time of the major peak(excluding the solvent peak) in the liquid chromatogram of the sample solution is the same as that of the standard solution obtained in the assay | The retention time of the major peak(excluding the solvent peak) in the liquid chromatogram of the sample solution is the same as that of the standard solution obtained in the assay | —— | —— | The retention time of the major peak(excluding the solvent peak) in the liquid chromatogram of the sample solution is the same as that of the standard solution obtained in the assay |
Assay(calculated with reference to the dried substance) | 98.0%-102.0% | 98.0%-102.0% | 98.0%-102.0% | 98.0%-102.0% | 98.0%-102.0% |
Mesh | —— | —— | —— | —— | —— |
Specific Rotation | +84.0°~ +87.5° | +84.0°~ +87.5° | +84.0°~ +87.5° | +84.0°~ +87.5° | +84.0°~ +87.5° |
Moisture | +84.0°~ +87.5° | +84.0°~ +87.5° | +84.0°~ +87.5° | +84.0°~ +87.5° | +84.0°~ +87.5° |
PH of 10% Aqueous Solution | —— | —— | —— | —— | 5-8 |
Methanol | 0.1% Max | 0.1% Max | ≤0.1% | 0.1% Max | 0.1% Max |
Arsenic (As) | —— | —— | 4mg/Kg Max | —— | 3mg/Kg Max |
Lead | 1mg/kg Max | —— | 1mg/kg Max | 1mg/kg Max | 1mg/kg Max |
Copper | —— | —— | —— | —— | 10mg/kg Max |
Iron | —— | —— | —— | —— | 10mg/kg Max |
Ignited Residue | 0.7% Max | 0.7% Max | ≤0.7% | 0.7% Max | 0.7% Max |
Hydrolysis Products (Chlorinated monosaccharides) | 0.1% Max | 0.1% Max | ≤0.16% | —— | 0.1% Max |
RelatedSub stance (Oth erchlorinated disacchar ides) | 0.5% Max | 0.5% Max | ≤0.5% | —— | 0.5% Max |
Heavy Metal | —— | 10mg/kg Max | —— | —— | 10mg/kg Max |
Triphenylphosphine oxide | —— | —— | 150mg/kg Max | —— | 150mg/kg Max |
Total Aerobic Count | —— | —— | —— | —— | 250cfu/g Max |
E.Coli | —— | —— | —— | —— | MPN/g<3.0 |
S.Aureus | —— | —— | —— | —— | Negative |
Salmonella | —— | —— | —— | —— | Negative |
Yeasts & Molds | —— | —— | —— | —— | 50cfu/g Max |
Sucralose has been widely used in medicine and health care.
Sucralose is an inert molecule that does not decompose, absorb or react with other components of the drug in the human body. The Joint FAO / WHO Expert Committee on food additives has determined sucralose as a class of food additive "gras". According to the conclusion of the European Food Safety Agency (EFSA), sucralose sweetener is safe and can be used in special medical food for children. As the domestic food and pharmaceutical enterprises continue to upgrade and update the formula, major enterprises have chosen sucralose as a sweetener.
The largest food and pharmaceutical enterprises in China have been adopting sucralose in increasing numbers as the primary sweetener in their constant search for new and better product formulas.
JK’s sucralose product has been approved as safe for use and consumption in over 3,000 differentiated food, pharmaceutical, and personal care products in many countries and continents around the world including the Americas, Europe, Japan, and India.
Pharmaceutical-grade sucralose can enhance the taste of medications when used in pharmaceuticals as an excipient while having no negative effects on the natural homeostasis of the human body, nor will it alter or diminish the effects of said medications.
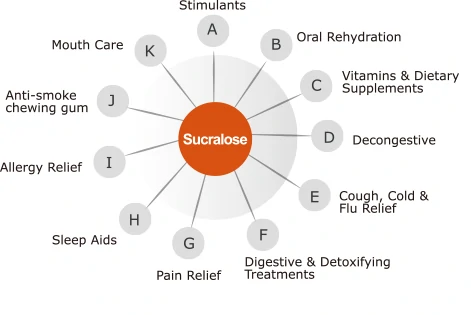
Thus, sucralose is used in a myriad of products such as medicinal syrups, oral liquid medications, sugar coating of pills, tablets, instant mix medications, capsules, cough medications, pain medications, rehydration fluids, sleep medications, antihistamines, nicotine gum, oral hygiene products, blood pressure medications, digestive medications, vitamins, and dietary supplements, etc. in order to replace sugar as a primary sweetening agent.

In the emerging pharmaceutical industry, sugar has been the preferred flavor enhancer and sweetener that is used; however, sugar can create a heavy load on the human body due to the negative health effects attributed to excess sugar and calorie consumption, and its ill effects on people with certain health conditions. Due to these health effects, pharmaceutical industries around the world have been seeking safe and stable, low-calorie sweeteners to replace sugar as a flavor enhancer.
Sucralose, with attributes including intense sweetness (roughly 600 times that of sugar by weight), smooth flavor, structural stability, and non-toxicity, is quickly becoming regarded as the pinnacle of non-sugar, zero-calorie sweeteners. On top of these attributes, sucralose does not cause tooth decay, affect blood insulin, sugar, or lipid levels in any way, and therefore, can be used by people with, and will not cause diabetes, cardiovascular disease and obesity. Because of all of these factors listed above, sucralose is fast becoming the most sought-after and preferred sweetener for healthcare supplements and medicinal products around the world, meeting all of the necessary demands of these products.
With its status as part of the latest generation of zero-calorie sweeteners, its competitiveness will grow exponentially with the increase in market demand for such sweeteners.

JK Sucralose Inc., located in Yancheng, Jiangsu Province, is the largest professional sucralose manufacturer in China and the second-largest in the world. JK Sucralose Inc. is the only Chinese company ever to voluntarily join the US ITC-337 Intellectual Property Rights investigation and eventually went on to win the case. JK has a long-term annual capacity target of 12,000 tons. JK is the only Chinese sucralose enterprise with direct-sales branches in the United States, Europe and Japan. It is one of the main enterprises setting the national standard for sucralose in China. JK has 20 independently owned processing patents in China and 4 independently owned processing patents in the US.




We are a professional manufacturer of Sucralose. Our product quality fully meets ISO9001, Kosher, HALAL, FAD and a number of international standards, such as FCCVII, USP34-NF29, EP2368 and other requirements.
◆ The only producer of pharmaceutical-grade sucralose in China.
◆ The only manufacturer to obtain the license to produce sucralose for use in pharmaceutical products.
◆ The sole sucralose producer with independently owned ISO Class 5 cleanroom pharmaceutical and GMP certified production facilities rated at 100,000 or fewer particles per m3.
We are a manufacturer, and we own a factory located in Sheyang County, Jiangsu, P.R. China. And it covers an area of about 600 mu.
TT, LC, OA, and DA, all the payments are optional, with the support of Chemball.
Of course! We specialized in this line for many years, many customers make a deal with me because we can deliver the goods on time and keep the goods top quality!
We understand most customers prefer stock, so we will try to keep stock for most products. However, there is no stock available for some of them, and it needs some time to synthesize.
Contact us by Chemball page, email, mobile phone, any way you like! We will try our best to respond ASAP!

