b

Thank You!
Your requirement has been sent, we will contact you quickly!
Sent Failed!
Try again!
[veterinary prescription drugs]
Product overview
Aureomycin premix is prepared from the whole fermentation broth of aureomycin producing bacteria and an appropriate amount of calcium carbonate.
Main components and molecular formula
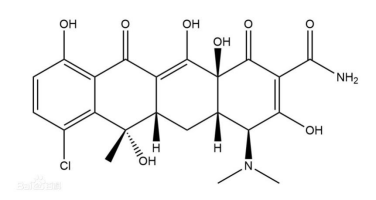
The main component is aureomycin (c22h23cln2o8, molecular weight: 478.88), and the structural formula is as follows:
Physical and chemical properties
The properties of aureomycin premix are brown or tan powder or particles, without agglomeration, mildew and odor. The melting point of aureomycin is 168 ~ 169 ℃ (decomposition), very slightly soluble in water and soluble in salt solution. Aureomycin hydrochloride is slightly soluble in methanol, water and ethanol, but insoluble in acetone, ether and chloroform. It is stable in the air and darkens when exposed to light.
Application effect
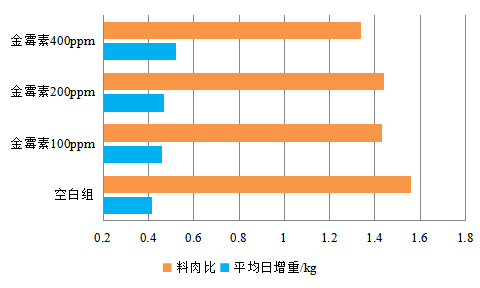
Figure 1: effect of aureomycin on growth performance of Weaned Piglets
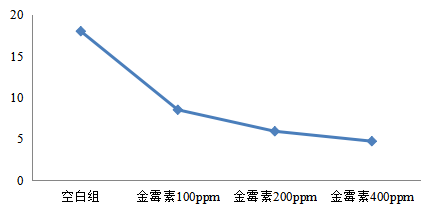
Figure 2: effect of aureomycin on diarrhea rate of Weaned Piglets
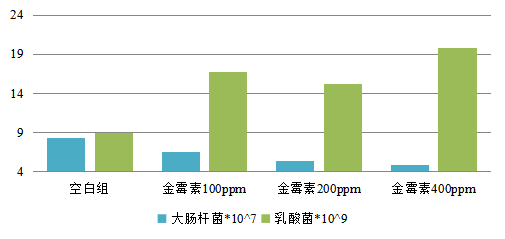
Figure 3: effect of aureomycin on intestinal flora of weaned piglets (CFU / g)
Usage method
Varieties | Mg / kg feed added |
(based on chlortetracycline) pig | 400-600 g for 7 days |
matters needing attention
*Aureomycin shall not be used 2 days before and 10 days after swine erysipelas vaccination.
*Aureomycin can form insoluble complexes with multivalent metal ions such as magnesium, calcium, aluminum, iron, zinc and manganese, thus affecting the absorption of drugs. Therefore, it should not be taken with drugs, feed and dairy products containing the above multivalent metal ions.
Drug withdrawal period
Pig :7DAY
Specification, packaging and storage
Specifications] Calcd for chlortetracycline 1000 μ G: 150 μ g (150 million units)
【 pack 】 25kg / bag, 1kg / bag
[storage conditions] shade, airtight, and preserved in a dry place.
【 approval No Veterinary Pharmacol 280, 114651
【 production unit 】 Gansu Huineng Bioengineering Co., Ltd

