b

Thank You!
Your requirement has been sent, we will contact you quickly!
Sent Failed!
Try again!
Triphosgene
I. Physical properties:
English name is Bis(trichloromethyl)carbonate, abbreviation is BTC. Molecular formula is C3Cl6O3, molecular weight is 296.75. Triphosgene is white to off-white crystal with phosgene odor. Melting point is 78-81℃, boiling point is 203--206℃; insoluble in water, easily soluble in chlorobenzene, toluene, dichloromethane, trichloromethane, and other organic solvents.
Structural formula:
.jpg)
II. Chemical properties:
Triphosgene can completely replace toxic phosgene and diphosgene in chemical reaction
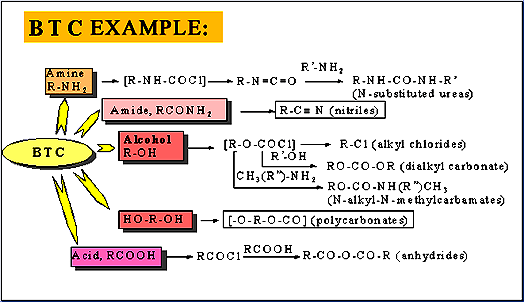
III. Advantages:
1) Safety in use, environmentally friendly.
2) Solid crystal is convenient to use, accurate measurement.
3) High yield of reaction product.
4) Can store and transport.
IV. Quality standard (Q/upchem 001-2000):78-81℃
Assay:99.0%, Melting point: 78-81℃
V. Packing:
1. Plastic drum, 30KG/drum
2. At the request of clients.
VI. Storage:
1. Keep in cool and well-ventilated place.
2. Separate from alkaline chemicals.
VII. Production capacity:
The annual production capacity of triphosgene is 5,000 tons, and the planned annaul production capacity is 20,000 tons.

