b

Thank You!
Your requirement has been sent, we will contact you quickly!
Sent Failed!
Try again!



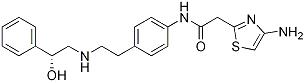
CAS:223673-61-8
Molecular formula: C21H24N4O2S
Molecular weight: 396.50
Appearance: white to off white powder
Content: more than 99%
Application: for the treatment of adult overactive bladder



