Triphosgene
I. Physical properties:
English name is Bis(trichloromethyl)carbonate, abbreviation is BTC. Molecular formula is C3Cl6O3, molecular weight is 296.75. Triphosgene is white to off-white crystal with phosgene odor. Melting point is 78-81℃, boiling point is 203--206℃; insoluble in water, easily soluble in chlorobenzene, toluene, dichloromethane, trichloromethane, and other organic solvents.
Structural formula:

II. Chemical properties:
Triphosgene can completely replace toxic phosgene and diphosgene in chemical reaction

III. Advantages:
1) Safety in use, environmentally friendly.
2) Solid crystal is convenient to use, accurate measurement.
3) High yield of reaction product.
4) Can store and transport.
IV. Quality standard (Q/upchem 001-2000):78-81℃
Assay:99.0%, Melting point: 78-81℃
V. Packing:
1. Plastic drum, 30KG/drum
2. At the request of clients.
VI. Storage:
1. Keep in cool and well-ventilated place.
2. Separate from alkaline chemicals.
VII. Production capacity:
The annual production capacity of triphosgene is 5,000 tons, and the planned annaul production capacity is 20,000 tons.
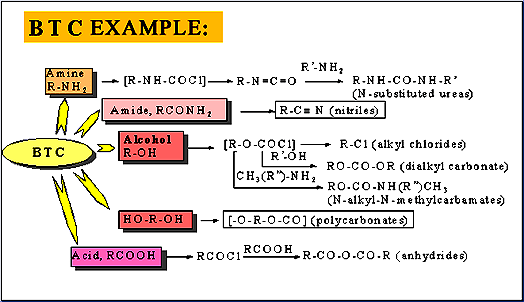
Triphoto gas (BTC), namely, bis (Trichloromethyl) carbonate, is commonly known as solid phosgene, and the chemical formula is c3cl6o3. It is colorless crystal, has the smell of light gas, mainly used as a substitute for phosgene, and is used in medicine, pesticide and organic synthesis, and has better effect than liquid double light gas. The trioptical gas is prepared by the reaction of dimethyl carbonate and chlorine gas. Three light gas at 130 ° C is decomposed around, and the presence of impurities reduces the temperature. There will also be a small amount of decomposition during atmospheric distillation to generate phosgene and double phosgene (Trichloromethyl chloroformate). When chloride exists, the phosgene can produce phosgene safely and quantitatively, which solves the problem that phosgene can not be quantified in the reaction. Triphosgene can produce almost all reactions of phosgene, and can be used as reagents for chloroformylation, chlorination, carbonylation and some polymerization reactions. It can convert primary amine into isocyanate or substituted urea, carboxylic acid into acyl chloride, alcohol into carbonate or aldehyde (with dimethyl sulfate), aldehyde oxime and amide into nitrile, etc.
IUPAC
bis(trichloromethyl) carbonate
SMILES
C(=O)(OC(Cl)(Cl)Cl)OC(Cl)(Cl)Cl




.jpg)