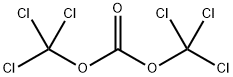
Bis(Trichloromethyl) Carbonate
Molecular formula: C3Cl6O3
Molecular weight: 296.75

Triphosgene is a white crystal, which decomposes in case of hot water and alkali hydroxide. The relative density is about 2, the boiling point is 205-206 ℃ (partial decomposition), and the melting point is 78-79 ℃. Soluble in ethanol, benzene and ether. Insoluble in water, soluble in ether, THF, benzene, cyclohexane, chloroform, carbon tetrachloride, 1,2-dichloroethane and other organic solvents. One molecule solid triphosgene can be decomposed into three molecule gas phosgene. Compared with gas phosgene, it has the advantages of transportation, safe use, convenient measurement, reverse dropping reaction and close to equivalent reaction. In industry, it is only treated as a general toxic substance. It can replace phosgene or diphospgene in medicine, pesticide, organic chemical industry and polymer synthesis.
The initial decomposition temperature of solid phosgene is 130 ℃, which begins to decompose at 90 ℃ in a humid atmosphere. It should be stored in a dry and cool place, away from the fire source, and separated from organic amines.
Uses: triphosgene is a good substitute for phosgene. It is widely used in the pharmaceutical industry to prepare chloroformyl compounds, such as intermediates of dozens of antibiotics such as aloxicillin, mezlocillin, peracillin and so on.
Health hazard: This product is a class II organic drug. It releases toxic gas in contact with water and causes burns in direct contact. Inhalation or ingestion is harmful to the body and has strong irritation to the eyes and respiratory tract. Severe cases can cause headache, dizziness, nausea and vomiting.
Emergency disposal: wash skin contact with a large amount of flowing water or soapy water. After contact with eyes, wash immediately with a large amount of water for 15 minutes and send to hospital. Those who ingest or inhale by mistake shall leave the scene quickly and go to a place with fresh air. They have difficulty breathing, take oxygen and seek medical treatment.
Protective measures: wear protective clothing, plastic coated gloves, gas mask or goggles when using or contacting this product.
Quality index
Project | index |
Appearance | White crystal |
Content% ≥ | 99.5 |
Melting point ℃ | 79-81 |
Loss on drying% ≤ | 0.5 |
Acidity% ≤ | 0.1 |
Packaging: (1) HDPE plastic barrel: 25kg / barrel, the inner PET plastic bag is bound and sealed, and the outer part is covered and sealed.
(2) Ton package: 730kg / package, the interior is bound and sealed with PET plastic bags, and there is a discharge port at the bottom (special requirements shall be subject to the agreement between the supplier and the buyer).
Storage: store in a cool, dry and well ventilated warehouse to avoid direct contact with water. The package must be sealed and not damp. It is forbidden to store together with alkali. Appropriate materials shall be prepared for storage to contain leakage. Meet the above storage conditions, and the shelf life is 24 months.
Triphoto gas (BTC), namely, bis (Trichloromethyl) carbonate, is commonly known as solid phosgene, and the chemical formula is c3cl6o3. It is colorless crystal, has the smell of light gas, mainly used as a substitute for phosgene, and is used in medicine, pesticide and organic synthesis, and has better effect than liquid double light gas. The trioptical gas is prepared by the reaction of dimethyl carbonate and chlorine gas. Three light gas at 130 ° C is decomposed around, and the presence of impurities reduces the temperature. There will also be a small amount of decomposition during atmospheric distillation to generate phosgene and double phosgene (Trichloromethyl chloroformate). When chloride exists, the phosgene can produce phosgene safely and quantitatively, which solves the problem that phosgene can not be quantified in the reaction. Triphosgene can produce almost all reactions of phosgene, and can be used as reagents for chloroformylation, chlorination, carbonylation and some polymerization reactions. It can convert primary amine into isocyanate or substituted urea, carboxylic acid into acyl chloride, alcohol into carbonate or aldehyde (with dimethyl sulfate), aldehyde oxime and amide into nitrile, etc.
IUPAC
bis(trichloromethyl) carbonate
SMILES
C(=O)(OC(Cl)(Cl)Cl)OC(Cl)(Cl)Cl